Back to the Basics : CHF vs. Chronic Bronchitis: Shortness of Breath

by Chris Ebright
Our articles are read by an automated voice. We offer the option to listen to our articles as soon as they are published to enhance accessibility. Issues? Please let us know using the contact form.
This initial installment of the Back to the Basics series is going to compare and contrast a common chief complaint: shortness of breath. Many etiologies provoke this, but many EMT students have a hard time differentiating a dyspneic congestive heart failure patient from one with chronic bronchitis. Both conditions present with physical similarities, coughing, wheezing, fatigue, and signs of hypoxia. Even though their chief complaint is identical, these patients develop dyspnea by different means. So, let’s compare the pathophysiology of each condition and see if we can clear this up, shall we?
Dyspnea with Chronic Bronchitis
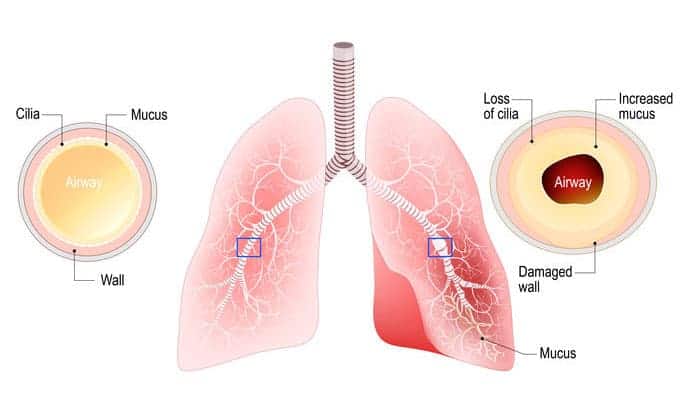
The chronic bronchitis patient suffers from constant inflammation of the lower airways, resulting in narrower-than-normal air passages. As a result, airflow volume decreases, and resistance to airflow increases, causing the patient to work harder to breathe, even while resting. The continuous inflammatory response triggers goblet cells to increase their mucus production significantly. Excessive mucus volume consequentially irritates the lower airway linings, inducing scar tissue development, and ciliary malfunction.
Cilia move mucus (and anything trapped within it) from the lower airways toward the upper airway - similar to how an escalator moves someone from a lower to a higher floor. This loss of “escalator function” allows mucus to remain within the inflamed, narrowed lower airway passages for extended periods; further narrowing the lumen and within some airways, blocking airflow altogether. Any pathogen trapped within the stale mucus proliferates, eventually invading local lung tissue and the surrounding bloodstream, perpetuating the immune response.
The air volume that reaches the alveoli is considerably less compared to a person without chronic bronchitis. The good news is, the alveoli are structurally intact, as is their wall elasticity. The bad news is, the low air volume minimally fills it, stretching the wall less than normal. The poor recoil during exhalation pushes out considerably lower air volume than what entered. This low volume, with a significantly lower pressure, cannot effectively traverse through the narrowed lower airways, causing abnormal alveolar retention. This is called air-trapping.
Since the bronchitis patient can only move a minimal amount of air volume to the alveoli, that also means that a minimal amount of oxygen is delivered. The decreased supply of available oxygen results in an inadequate amount that can be delivered into the pulmonary circulation, the left heart, and the body. Over time, the progressive decrease in oxygen concentration lowers the oxygen alveolar pressure, i.e., the gas has decreased “strength” to traverse the respiratory membrane and enter the bloodstream. Consequently, oxygen is further retained within the alveoli and doesn’t enter circulation.
The generated hypoxemia (lack of oxygen in the blood) increases the volume of circulating carbon dioxide. The concentrated gas easily crosses the respiratory membrane – rapidly taking up alveolar space, allowing even less room for oxygen, and further decreasing its alveolar pressure. As alveolar carbon dioxide concentration increases (because the alveoli cannot effectively expel air), alveolar pressure ultimately exceeds the serum pressure of carbon dioxide. It remains retained within the bloodstream, passing through the pulmonary circulation and right back into the systemic circulation, eventually causing acidosis.
Hypoxemia eventually leads to hypoxic tissues and organs - particularly the brain. It responds by increasing the rate of red blood cell production. As the volume of red cells increases, the serum plasma level diminishes, tremendously increasing the blood’s viscosity. Sluggish blood flow and ever-dwindling oxygen delivery cause the brain to continue the overstimulation of red blood cell production - perpetuating a patient’s overall deterioration.
Eventually, the pressure required to circulate syrupy blood through pulmonary circulation becomes higher than the right ventricle can generate (pulmonary hypertension). The overload causes right ventricular hypertrophy and failure (cor pulmonale) and eventually induces retrograde blood flow into the venous system. As this increasing back-pressure is transmitted through the inferior vena cava and all points distal, serum fluid is third-spaced into the liver, spleen, the peritoneal cavity (ascites), and the lower extremities.
So, the chronic bronchitis patient is constantly hypoxemic and hypoxic; acidotic; has poor right-sided heart function, pulmonary, and systemic perfusion (manifesting as low exercise/movement tolerance); a large belly that reduces thoracic cavity volume, plus the patient is prone to sitting more than standing from being constantly hypoxic = tired; and is susceptible to recurring infections because of poor circulation and a taxed immune system.
When EMS is called for a chronic bronchitis patient with dyspnea, it is a worsening of these predisposing conditions. Basic management focuses on reversing the acute hypoxia, bronchoconstriction, and inflammation by assisting with the administration of bronchodilators (albuterol) and oxygen.
Dyspnea with Congestive Heart Failure
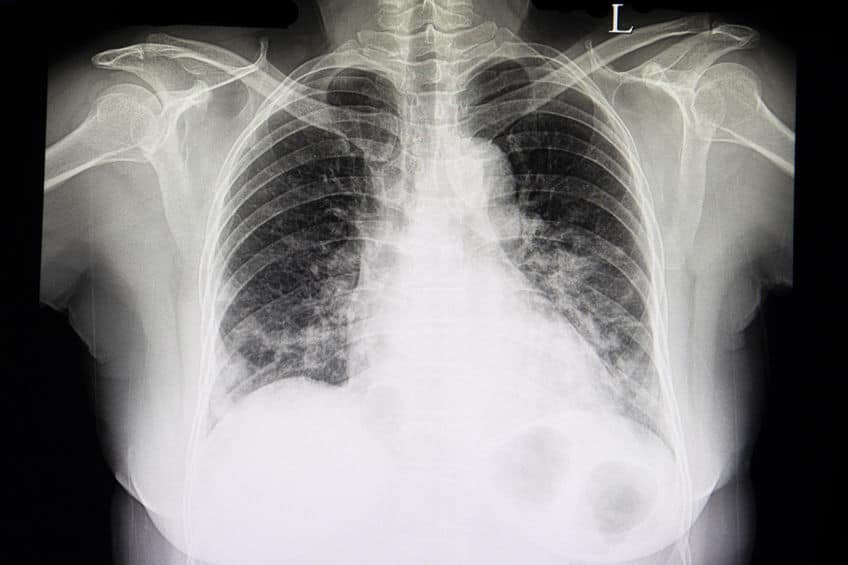
A chest x-ray film of a patient with cardiomegaly, congestive heart failure, and pulmonary edema.
Congestive heart failure (CHF) is a consequence of an abnormality in cardiac structure, function, rhythm, or conduction. Hypertension, infection, a massive myocardial infarction or the cumulation of smaller infarcts, disease of the atrioventricular valves and/or the semilunar valves, and diseases of the heart muscle (cardiomyopathies) are typical etiologies. Some conditions cause atrophy (diminish the size) of the ventricular wall, while others excessively enlarge (hypertrophy) the wall. Regardless, the initial insult is usually to the left ventricle, eventually causing it to become an inefficient pumping chamber.
Typically, the left ventricular wall hypertrophies as compensation from increased afterload, or by direct damage from a myocardial infarct secondary to coronary artery disease. The Frank-Starling law of the heart states that as ventricular volume increases and stretches the myocardial muscle fibers, the stroke volume increases proportionally. A hypertrophied left ventricle, though, has the consistency of concrete (so to speak) that grossly diminishes its stretching ability, thus negatively affecting output. Additionally, the hypertrophied wall expands into the ventricular chamber, dwindling the amount of available space.
The reduced filling space decreases the available blood volume wall to stretch the myocardial muscle fibers, and the inflexible, rigid wall produces insufficient pressure to force adequate volume through the aorta. The result is a decrease in cardiac output, which compromises not only systemic circulation, but also coronary circulation. The left ventricle has now failed as a forward pump.
The openings for the left and right coronary arteries are just superior to the aortic semilunar valve, which closes during ventricular diastole. When this valve closes, the blood remaining within the aorta flows back toward it, filling the coronary arteries. As CHF progresses, the associated decrease in aortic blood volume, consequently curtails coronary artery filling. Less blood and nutrients become available to the myocardium, perpetuating the heart failure.
A hormone release as a response to the decrease in cardiac output causes more problems. Activation of the renin-angiotensin system stimulates renal retention of salt and water, thus, increasing circulating blood volume. Activation of this system also initiates systemic vasoconstriction, increasing the resistance (afterload) against which the failing heart must pump.
The left ventricle becomes even less efficient as a forward pump. The excessive retained volume trying to pass through a narrow ventricle chamber, combined with an increased afterload causes a ventricular diastolic pressure increase. This pressure cannot be effectively reduced by pumping volume through the aorta, so it back-builds into the left atrium, eventually dilating and thinning the left atrial wall. Thinner in comparison to the left ventricular wall, it cannot withstand increased pressure nearly as long, and swiftly fails. The left-sided diastolic pressure continues to escalate, leading to pulmonary venous congestion.
As the venous pressure increases, pulmonary capillaries and lymphatic vessels are recruited to deal with the added volume, essentially providing extra drainage avenues to keep the pulmonary circulation pressure "normal." However, as the pressure continues climbing, these mechanisms fail, and the patient develops pulmonary hypertension.
An acute exacerbation of left ventricular failure induces a spike in left diastolic pressure, consequently increasing pulmonary capillary pressure. The result is excessive leakage of serum fluid, proteins, and occasionally red blood cells (pulmonary edema), into the interstitial lung tissue and alveoli. Crackles, wheezing, coughing, and shortness of breath typically manifest as signs and symptoms.
A congestive heart failure patient’s hypoxia and call to EMS for shortness of breath arise from a ventilation/perfusion mismatch. Outside of infection, when a CHF exacerbation occurs, air will effectively flow through the upper and lower airways. Past this point, oxygen cannot effectively traverse into the bloodstream through the fluid-filled alveoli. Ergo, carbon dioxide is retained within the bloodstream for the same reason. Hypoxemia, hypoxia, and acidosis develop, causing similar symptoms seen with chronic bronchitis.
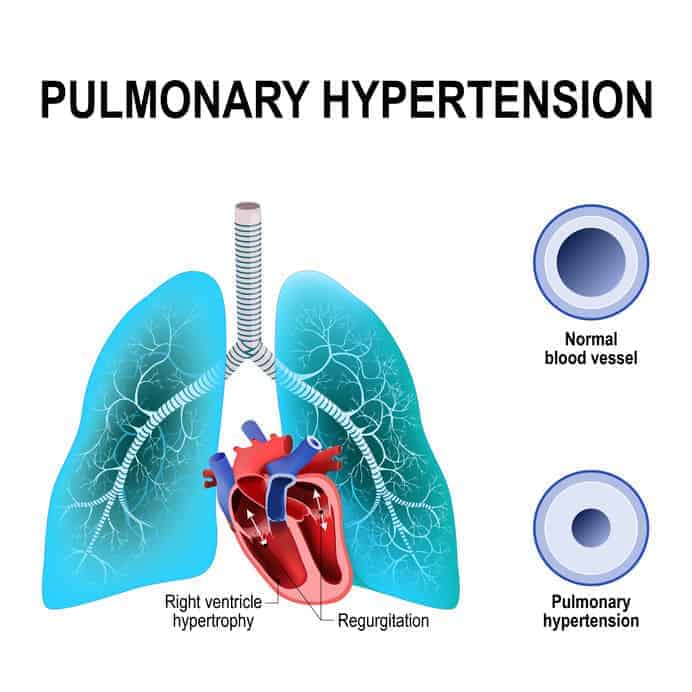
Left-sided failure and pulmonary hypertension eventually cause the right side of the heart to fail in many CHF patients. The consequence of a similar series of events - retrograde venous blood flow, increasing back-pressure, etc., as seen with the chronic bronchitis patient assessment. In contrast, however, the basic CHF treatment regimen focuses on fluid removal and reduction of pulmonary and systemic afterload. This is accomplished by the application of CPAP and assisting with the administration of sublingual nitrates. Occasionally, diuretics at the advanced level of care are administered to release additional fluid from the body.
Summing up, COPD and CHF are severe conditions that affect a patient’s breathing, but have different origins - COPD affects the lungs and CHF affects the heart. Both conditions present with physical similarities, symptoms, and risk factors. However, the EMT must recognize that a thorough history and physical exam may allow an accurate differentiation, but may not always be possible, as these two conditions can exist simultaneously.
Related articles

Dan Limmer, BS, NRP

Limmer Education
Comments
Leave a commentvery nice explanations in chronic bronchitis and CCF. Thank you so much.
This article was great! Very informative and the photos help conceptualize the process of each deteriorating condition.